AHRI’s clinical trial researchers, who are part of the wider STREAM Trial – a multi-country clinical trial that seeks to examine shortened regimens for multidrug-resistant tuberculosis (MDR-TB), successfully publish the STREAM Stage 2 Trial findings on THE LANCET.
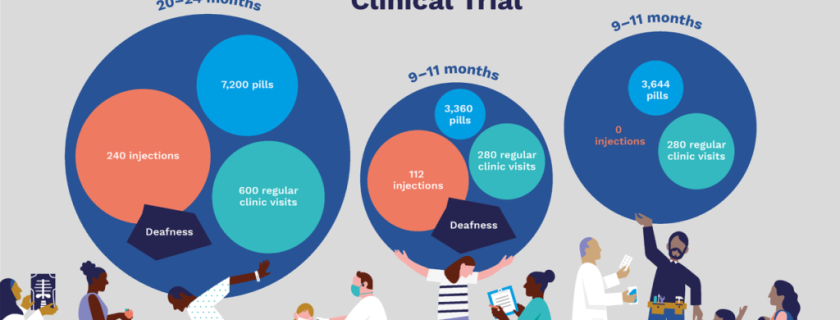
Over the years, significant strides have been made in the MDR-TB treatment landscape. In early 2012, when the STREAM trial began, the standard of care for MDR-TB lasted up to 24 months, and included an injectable agent which had an average success rate of just over 50%.
Initially, Stage 1 aimed to generate evidence to support the use of an effective new regimen that would significantly reduce treatment time for MDR-TB. When bedaquiline received US FDA approval in December 2012, it also offered a possible alternative to regimens containing injectable agents, which were associated with significant side effects, including hearing loss and renal impairment. Thus, Stage 2 of the trial was initiated to generate evidence regarding the efficacy of shorter, bedaquiline-containing regimens.
The STREAM Stage 2 Trial which began in 2016, with thirteen trial sites in Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, and Uganda; sought to evaluate the efficacy, safety, and cost of a 9-month all-oral, bedaquiline-containing regimen vs. the 9-month injectable-containing regimen evaluated in Stage 1.
The STREAM Stage 2 Trial findings have now shown that both bedaquiline-containing regimens, a 9-month oral regimen and a 6-month regimen with 8 weeks of second-line injectable, had superior efficacy compared with a 9-month injectable-containing regimen, with fewer cases of hearing loss.
For in-depth insights on the published article on THE LANCET, click the link below.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)02078-5/fulltext